|




| 货号 | LB279 |
| 品牌 | LB |
| 用途 | 科研 |
| 外观 | 白色结晶粉末 |
| 包装规格 | 25KG/桶 |
| 纯度 | 99% |
| CAS编号 | |
| 别名 | 匹可硫酸钠 |
| 保质期 | 36月 |
| 级别 | 医药级 |
| 质量标准 | USP43 |
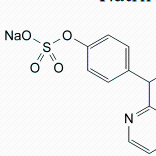
C18H13NNa2O8S2,H2O
Mr 499.4
DEFINITION
Sodium picosulfate contains not less than 98.5 per centand not more than the equivalent of 100.5 per cent of4,4′-(pyridin-2-ylmethylene)bisphenyl bis(sodium sulphate),calculated with reference to the anhydrous substance.CHARACTERSA white or almost white, crystalline powder, freely soluble inwater, slightly soluble in alcohol.IDENTIFICATIONFirst identification: A, E.Second identification: B, C, D, E.A. Examine by infrared absorption spectrophotometry(2.2.24), comparing with the spectrum obtained withsodium picosulfate CRS. Examine the substancesprepared as discs.B. Examine the chromatograms obtained in the test forrelated substances in ultraviolet light at 254 nm. Theprincipal spot in the chromatogram obtained with testsolution (b) is similar in position and size to the principalspot in the chromatogram obtained with referencesolution (a).C. To 5 ml of solution S (see Tests) add 1 ml of dilutehydrochloric acid R and heat to boiling. Add 1 ml ofbarium chloride solution R1. A white precipitate isformed.D. To about 10 mg add 3 ml of sulphuric acid R and 0.1 mlof potassium dichromate solution R1. A violet colourdevelops.E. The solution S gives reaction (a) of sodium (2.3.1).TESTSSolution S. Dissolve 2.5 g in distilled water R and dilute to50 ml with the same solvent.Appearance of solution. Solution S is clear (2.2.1) and notmore intensely coloured than reference solution GY7 (2.2.2,Method II).Acidity or alkalinity. To 10 ml of solution S add 0.05 ml ofphenolphthalein solution R. The solution is colourless. Notmore than 0.25 ml of 0.01 M sodium hydroxide is requiredto change the colour of the indicator to pink.Related substances. Examine by thin-layer chromatography(2.2.27), using silica gel GF254 R as the coating substance.Test solution (a). Dissolve 0.20 g of the substance to beexamined in methanol R and dilute to 5 ml with the samesolvent.Test solution (b). Dilute 1 ml of test solution (a) to 10 mlwith methanol R.Reference solution (a). Dissolve 20 mg of sodiumpicosulfate CRS in methanol R and dilute to 5 ml with thesame solvent.Reference solution (b). Dilute 2 ml of test solution (b) to100 ml with methanol R.Reference solution (c). Dissolve 0.20 g of the substance tobe examined in 2 ml of a 103 g/l solution of hydrochloricacid R. Heat rapidly to boiling and maintain boiling for 10 s.Cool in iced water and dilute to 10 ml with methanol R.Apply to the plate 5 μl of each solution. Develop over apath of 10 cm using a mixture of 2.5 volumes of anhydrousformic acid R, 12.5 volumes of water R, 25 volumes ofmethanol R and 60 volumes of ethyl acetate R. Dry theplate in a current of hot air for 15 min and examine inultraviolet light at 254 nm. Spray with a 200 g/l solution ofhydrochloric acid R in methanol R and heat at 110 °C for10 min. Spray the hot plate with a freshly prepared solutioncontaining 50 g/l of ferric chloride R and 1 g/l of potassiumferricyanide R. Examine the wet plate. Any spot in thechromatogram obtained with test solution (a), apart fromthe principal spot, is not more intense than the spot in thechromatogram obtained with reference solution (b) (0.2 percent). The test is not valid unless the chromatogram obtainedwith reference solution (c) shows three clearly separatedspots. A fourth spot may be present on the starting-line.Chlorides (2.4.4). Dilute 5 ml of solution S to 15 ml withwater R. The solution complies with the limit test forchlorides (200 ppm).Sulphates (2.4.13). Dilute 7.5 ml of solution S to 15 ml withdistilled water R. The solution complies with the limit testfor sulphates (400 ppm).Heavy metals (2.4.8). 12 ml of solution S complies with limittest A for heavy metals (20 ppm). Prepare the standard using10 ml of lead standard solution (1 ppm Pb) R.Water (2.5.12): 3.0 per cent to 5.0 per cent, determined on0.500 g by the semi-micro determination of water.ASSAYDissolve 0.400 g in 80 ml of methanol R. Titrate with0.1 M perchloric acid, determining the end-pointpotentiometrically (2.2.20).1 ml of 0.1 M perchloric acid is equivalent to 48.14 mg ofC18H13NNa2O8S2.